Absorption and Emission of Photons by Atoms
An atom absorbs or emits photons whenever one of its electrons makes a transition between two quantized energy levels. The basic idea is straightforward, although its implications are central to quantum physics.
A brief reminder
In a quantum atom, electrons are negatively charged particles bound to the nucleus and confined to discrete energy levels. Each level corresponds to an orbital with a characteristic shape and orientation, known as a subshell.
The energy levels closest to the nucleus are the lowest in energy, while the outer levels correspond to higher energies.
With this in mind, the mechanisms behind photon absorption and emission become easier to follow.
Absorption of a photon
When an atom is exposed to electromagnetic radiation, photons in the incoming beam may interact with its electrons.
During such an interaction, energy is transferred to the electron. Put simply, the electron absorbs the energy carried by the photon.
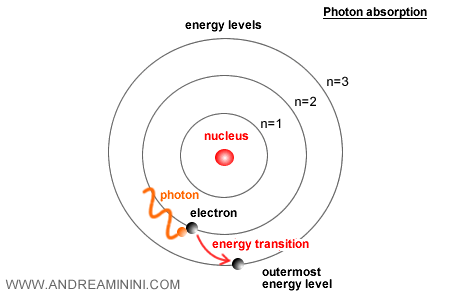
Once the electron has gained enough energy, it becomes excited and is promoted to a higher, more distant energy level (an upward transition).
What is an excited electron? It is an electron that has absorbed external energy, for example from a photon, and has moved into an excited state.
The electron can reach this excited state only if the absorbed energy matches or exceeds the energy gap separating the two quantized levels.
Can an excited electron leave the atom? Yes. If the absorbed energy surpasses the ionization energy, the electron escapes the atom entirely, becoming a free particle. The atom is then left as a positive ion.
Emission of a photon
After a brief time, an excited electron naturally loses energy. Pulled by the electrostatic attraction of the nucleus, it eventually drops back to a lower energy level (a downward transition).
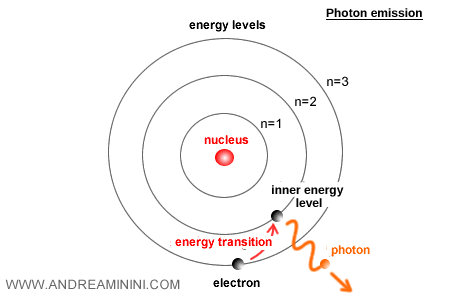
The lower level requires less energy than the excited one. As the electron settles into this new orbital, it must release the excess energy, which is emitted as a photon.
After releasing this energy, the electron returns to its ground state.
Note. In a quantum atom, only certain states are allowed, called stationary states. An electron cannot move toward the nucleus in a continuous way. Instead, it must jump between discrete energy levels, releasing a specific quantum of energy. This principle lies at the heart of quantum mechanics and underpins the Bohr model of the atom.
The downward transition to a lower level is the moment when the atom emits a photon.
Only during such a transition does the electron radiate energy.
Electrons do not radiate energy while they remain in a stationary state around the nucleus. Radiation occurs exclusively during downward transitions between quantized levels.
