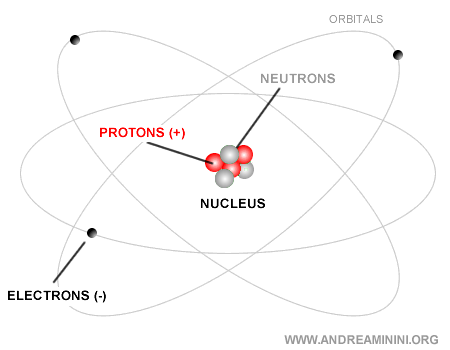
Atomic Nucleus
The atomic nucleus is the compact core at the center of an atom, tiny compared to the atom’s overall size. It consists of protons, which carry a positive charge, and neutrons, which are electrically neutral. The nucleus contains almost all of the atom’s mass and is held together by the strong nuclear force.
The nucleus itself is built from just two types of subatomic particles: protons and neutrons.
- Protons. Positively charged subatomic particles. A proton has a mass of 1.6726·10-27 kg and a positive electric charge of +1.6022·10-19 C.
- Neutrons. Electrically neutral subatomic particles. A neutron has a mass of 1.6749·10-27 kg - slightly heavier than a proton. Since it has no charge, it is called a neutron.
Together, protons and neutrons are known as nucleons. A classic model of the atom is shown below:
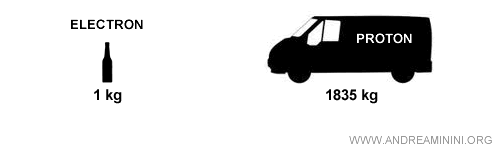
Note. Protons and neutrons are vastly more massive than electrons - about 1835 times heavier. An electron has a mass of 9.109534·10-31 kg, while protons and neutrons are on the order of 1.6·10-27 kg. To picture the difference: if an electron were the size of a one-liter bottle, a proton would be the size of a delivery van.
The Size of the Nucleus
The nucleus occupies only a minute fraction of the atom’s space compared with the electron cloud.
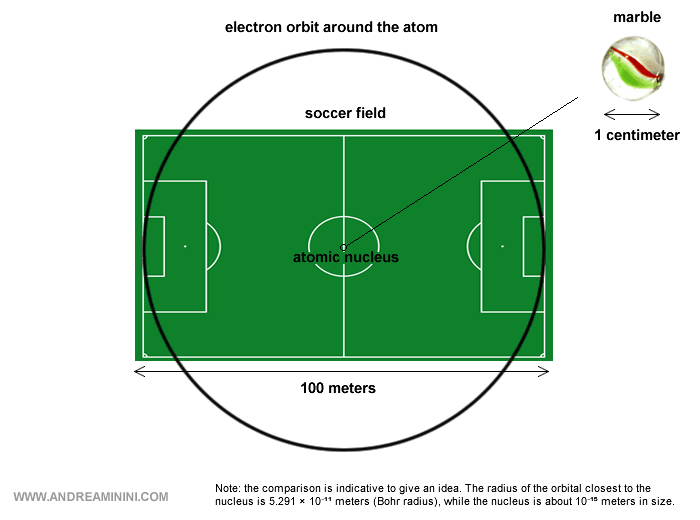
The nucleus measures about 10-15 meters across - roughly ten thousand times smaller than the atom itself, which is about 10-11 meters.
Charge of the Atomic Nucleus
The nucleus always carries a positive charge.
This charge is due entirely to the protons, since neutrons have no charge and play no role in this respect.
Each proton has a charge of +1.6022 x 10-19 C, which means the nucleus as a whole is positively charged.
In a neutral atom, the nucleus’s positive charge is exactly balanced by the negative charge of the orbiting electrons.
Note. An electron carries the same amount of charge as a proton, but with the opposite sign: -1.6022 x 10-19 C. This is why, in a neutral atom, the number of protons always equals the number of electrons.
Who Discovered Protons?
The idea of the proton was first put forward by Ernest Rutherford in 1911. At that time, electrons had already been observed, but the nucleus had not yet been identified.
Since electrons are negatively charged but atoms are overall neutral, Rutherford reasoned there must be other particles with equal and opposite (positive) charge to balance them out.
He therefore proposed the existence of protons within the atomic nucleus.
Note. Experiments with cathode rays had already shown that positively charged particles exist, but Rutherford was the first to suggest that the nucleus is specifically composed of protons.
Who Discovered Neutrons?
Following Rutherford’s work, scientists began making precise measurements of subatomic masses.
The results didn’t add up. The combined mass of protons and electrons fell short - accounting for only about half the atom’s total mass.
This led to the idea of another particle in the nucleus: one with a mass similar to a proton but no electric charge.
This particle was named the neutron.
The prediction proved correct. In 1932, British physicist James Chadwick provided the first experimental evidence of neutrons in the atomic nucleus.
Average lifetime of a neutron. A free neutron - that is, one not bound inside a nucleus - has an average lifetime of about 904 seconds. After that, it decays into a proton and an electron. Inside the nucleus, however, a neutron bound to protons is stable indefinitely.
How Many Neutrons Are in the Nucleus?
Once you know the atomic number (Z) and the mass number (A), you can determine the number of neutrons (N) simply by subtraction:
N = A - Z
Note. The atomic number (Z) is the number of protons in the nucleus. The mass number (A) is the total number of nucleons (protons + neutrons).
For lighter elements (roughly Z < 30), the number of neutrons is usually equal to the number of protons.
Still, there are stable atoms that share the same atomic number but have different mass numbers. These are known as isotopes.
What Is a Nuclide?
The possible combinations of atomic number (Z), mass number (A), and energy state that exist in nature are called nuclides.
A nuclide is simply the nucleus treated as a distinct physical entity.
Note. Nuclides with the same atomic number but different numbers of neutrons are isotopes.
The Nuclear Force (Strong Interaction)
Nucleons are bound together in the nucleus by the strong nuclear force.
This force is extraordinarily powerful but acts only over an extremely short distance - about 10-15 meters.
Note. The strong force is vastly stronger than the Coulomb repulsion, which would otherwise push the positively charged protons apart.
The energy that holds the nucleons together is called binding energy.
