Pauli Principle
What the Pauli Principle Says
Two electrons can occupy the same orbital only if they have opposite spins.
This is why it is also known as the exclusion principle: it rules out the possibility of two electrons sharing the exact same quantum state.
Since spin can take only two values (up or down), any given atomic orbital can hold no more than two electrons.

For this reason, the Pauli principle is sometimes informally described as the electron pairing rule.
It stands as one of the fundamental guidelines for determining the electronic configuration of atoms.
What is spin? Spin is one of the electron’s fundamental quantum numbers. It reflects an intrinsic property of the particle that can take two possible values: spin up (+½) and spin down (−½). Although often pictured as a particle “spinning” on its axis, spin is in fact a purely quantum property with no direct classical counterpart.
More broadly, the Pauli exclusion principle applies to all fermions - particles with half-integer spin that occupy antisymmetric quantum states.
No two identical fermions can ever occupy the same quantum state simultaneously.
This extends the principle beyond electrons to include protons, neutrons, and all other fermions.
Formulated in 1925 by Austrian physicist Wolfgang Pauli, it remains one of the cornerstones of modern quantum mechanics.
A practical example
The lithium atom has atomic number Z = 3.
That means it contains three electrons.
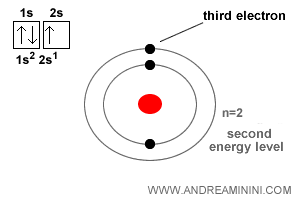
The first two electrons fill the innermost spherical orbital (1s) at the lowest energy level, n=1.
The third electron moves into the next spherical orbital (2s), at the higher energy level n=2.
In atoms, electrons always occupy the lowest available orbitals first, then progressively fill those of higher energy.
Note. In an orbital diagram, each box represents an orbital, and each arrow an electron. The direction of the arrow indicates spin orientation - up or down. Thus, within a single orbital, you can never have two arrows pointing in the same direction (both up or both down).

Example 2
The beryllium atom has atomic number Z = 4.
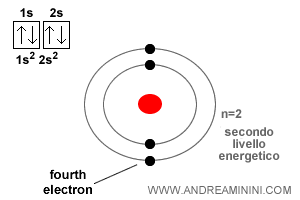
The first two electrons occupy the 1s orbital, the innermost and lowest in energy. The third electron enters the 2s orbital, just as in lithium.
The fourth electron also goes into the 2s orbital, but only because it can take the opposite spin to the one already present.
This is precisely what the Pauli exclusion principle guarantees: an orbital can hold no more than two electrons, and when it does, their spins must be opposite.
And so on.
