Fermions
Fermions are fundamental particles that obey the Pauli exclusion principle, which states that no two identical fermions can occupy the same quantum state simultaneously.
All particles in the universe fall into one of two broad categories: fermions and bosons.
Fermions make up matter itself - everything you can touch or see is composed of them. They form the atoms and molecules that structure the physical world.
Why are they called fermions?
The term “fermion” honors Italian physicist Enrico Fermi, as these particles follow Fermi-Dirac statistics, which describe how they populate different energy levels.
Note. In 1926, Fermi introduced a statistical model to describe particles that obey the exclusion principle - that is, particles that cannot share the same quantum state. Paul Dirac later refined this theory, and it became known as Fermi-Dirac statistics. The particles governed by it are now called fermions, in recognition of Fermi’s contribution.
Fermions fall into two main categories: quarks and leptons.
- Quarks are the constituents of protons and neutrons.
- Leptons include particles such as the electron and the neutrino.
Baryons - which include protons and neutrons - are also classified as fermions.
What defines a fermion?
The key property of fermions is their spin, which is always a half-integer value, such as ±1/2, ±3/2, and so on.
What is spin? Spin is an intrinsic quantum property with no direct classical analogue, though it's often loosely described as a kind of “internal rotation” of the particle.
Every fermion is subject to the Pauli exclusion principle.
This means that no two identical fermions can occupy the same quantum state in the same location at the same time.
For instance, electrons in an atom are distributed across orbitals with different energy levels around the nucleus. Within a given orbital, only two electrons can coexist - and only if their spins are opposite: one “up,” one “down.”

Take the lithium atom, which contains three electrons. The first two occupy the lowest-energy orbital near the nucleus - one spin-up, the other spin-down. The third is forced into the next available orbital, slightly farther out.
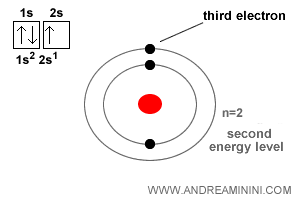
This fundamental “no-overlap” rule is why matter has structure and stability. Without it, all matter would collapse into a single dense state.
What’s the difference between fermions and bosons?
Fermions and bosons are the two basic classes of elementary particles. Their main distinction lies in their spin - and in how they behave under quantum statistics.
- Fermions
Fermions have half-integer spin (e.g., 1/2, 3/2, etc.). Electrons, protons, and neutrons are all fermions. They follow the Pauli exclusion principle, meaning no two identical fermions can share the same quantum state. This property underlies the structure of atoms, chemistry, and ultimately all solid matter. - Bosons
Bosons have integer spin (0, 1, 2, etc.) and act as force carriers. For example, photons mediate the electromagnetic force, while gluons are responsible for the strong nuclear force binding quarks together in protons and neutrons. Unlike fermions, bosons can occupy the same quantum state - meaning they can “pile up” in the same place and state without restriction. They do not obey the Pauli exclusion principle.
This distinction is fundamental to quantum physics and has profound implications - from the structure of matter to the workings of the universe’s fundamental forces.
