Wave - Particle Duality
Wave - particle duality describes the twofold nature of matter and radiation: in some experiments they behave like discrete particles, while in others they act as waves capable of interference and diffraction.
This principle lies at the heart of quantum mechanics: both matter and radiation simultaneously display wave-like and particle-like properties.
Phenomena such as interference and diffraction reveal their wave character, while localized collisions and energy quanta highlight their particle aspect.
In modern physics, these two manifestations are unified through the wave function.
Note. The double-slit experiment is the most famous demonstration of wave - particle duality in matter and radiation. It shows that photons and electrons can exhibit either wave-like or particle-like behavior, depending on how they are observed.
Double-Slit Experiment
In the 18th century, the dominant theory, strongly backed by Isaac Newton, held that light was composed of minute “corpuscles” streaming from luminous objects.
In 1801, however, Thomas Young’s double-slit experiment persuaded scientists of its wave-like nature.
Young passed a beam of light through two parallel slits cut into an opaque screen.
On the panel placed behind the slits, instead of the two bright bands that had been expected, an alternating pattern of light and dark fringes appeared - an unmistakable signature of wave interference.
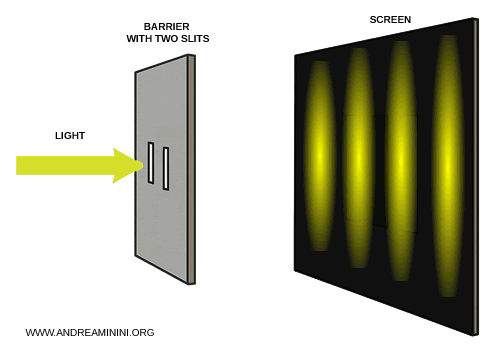
Light was clearly acting like a wave, capable of overlapping to produce constructive and destructive interference.
- At points of constructive interference, the waves combine to increase intensity.
- At points of destructive interference, the waves cancel each other, reducing or even eliminating intensity.
Seen from above, one can observe regions where the waves overlap and reinforce each other (red points) and others where they cancel out, producing multiple vertical bars on the screen.
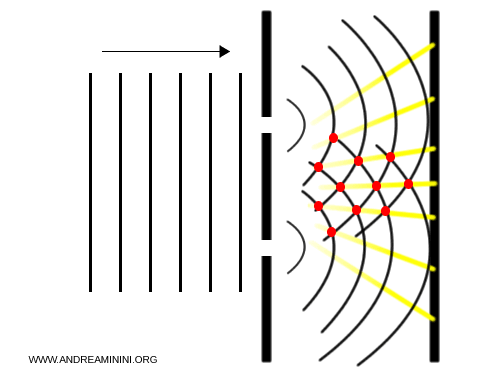
This result led 19th-century scientists to conclude that light - and electromagnetic radiation in general - was purely wave-like, and the particle hypothesis was abandoned.
The Return of the Particle Hypothesis
The particle view returned in the early 20th century with Max Planck’s quantum theory, which introduced the idea of quantized energy: divided into minimal, discrete packets (quanta).
In 1905, Albert Einstein applied quantum theory to explain the photoelectric effect, proposing that light consists of indivisible particles called photons.
From then on, light came to be seen as having a dual identity: both particle (photons) and wave.
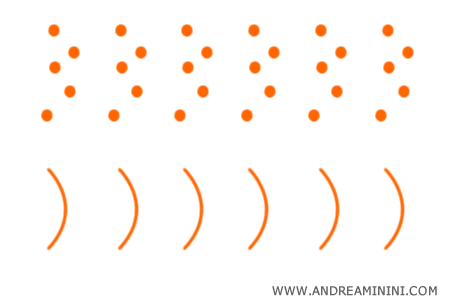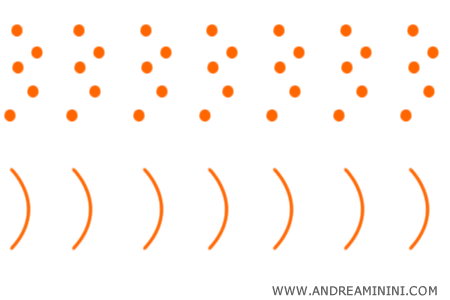
In 1924, Louis de Broglie extended the idea by suggesting that electrons also possess a dual wave - particle character.
Wave - particle duality was no longer limited to radiation - it applied to matter itself.
Wave - Particle Duality of Electrons
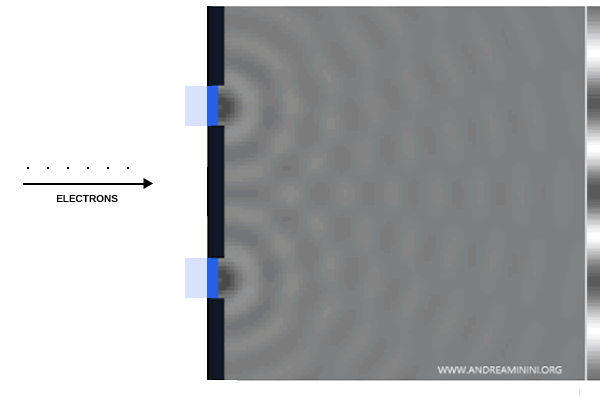
Young’s experiment was repeated, this time with streams of electrons fired at a barrier containing two slits.
If electrons behaved strictly as particles, one would expect to see two sharp bands on the screen, corresponding to the two slits.
Instead, the screen revealed an interference pattern identical to that of light: each electron seemed to pass through both slits at once, behaving like a wave.

To eliminate the possibility of interactions between electrons, the experiment was repeated with electrons sent through one at a time.
Remarkably, even then the same interference pattern emerged, confirming the wave-like nature of electrons.
But when a detector was introduced to determine which slit an electron passed through, the interference pattern vanished, leaving only two distinct bands - just as if the electrons were ordinary particles.
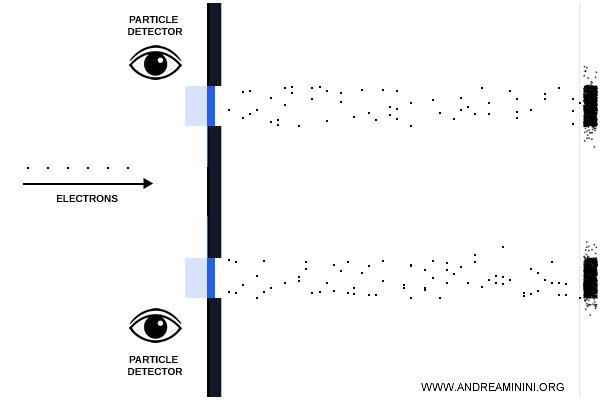
This experiment shows that:
- without observation, electrons behave like waves, producing interference patterns;
- with observation, they behave like localized particles, leaving only two traces.
Quantum mechanics explains this through the wave function, which encodes the probability of finding a particle at a given location. The act of observation causes the wave function to “collapse,” eliminating superposition and yielding a definite outcome.
Note. To find out which slit a particle passes through, physicists use devices such as photodiodes or electronic sensors that can register the passage of a photon or electron, or employ scattering techniques that interact with the particle along its path. The key point is that any attempt to determine the particle’s path inevitably introduces an interaction. That interaction erases the interference pattern and shifts the particle’s behavior from wave-like to particle-like.
This means that light - and matter more broadly - cannot be described solely as waves or solely as particles. Instead, they possess a dual wave - particle nature (wave - particle duality) that reveals itself differently depending on the kind of measurement or experiment performed.
And so the story continues.
