Beta decay ( β )
Beta decay is a type of nuclear transformation governed by the weak interaction. In this process, a neutron can turn into a proton (β⁻) or a proton into a neutron (β⁺) through a change in quark flavor.
During the transformation, a virtual $W$ boson is emitted. It almost immediately decays into an electron and an electron antineutrino (β⁻), or into a positron and an electron neutrino (β⁺).
This process alters the balance between protons and neutrons, enabling the nucleus to reach a more stable configuration.
When does it occur?
Beta decay occurs whenever the nucleus contains either too many or too few neutrons relative to protons.
In such cases, the weak interaction converts a neutron into a proton (β⁻) or a proton into a neutron (β⁺), restoring a more stable balance.
β⁻ decay can also happen in free neutrons, which are unstable outside the nucleus and spontaneously decay into protons.
Note. Beta decay also produces beta radiation, consisting of electrons (β⁻) or positrons (β⁺), accompanied by a neutrino or antineutrino. This radiation carries away the excess energy released during the nuclear transformation.
β- decay
When a nucleus has an excess of neutrons, it can stabilize by converting a neutron into a proton.
How does a neutron become a proton?
On the subatomic scale, this happens when a down quark inside the neutron transforms into an up quark.

When a down quark $(-\tfrac13 e)$ interacts with the weak field (the W field), it can change into an up quark $(+\tfrac23 e)$, increasing its charge by $+1e$.
To conserve the total charge of the system, a virtual boson $W^-$ is emitted at the same time. Since it carries an electric charge of $-1e$, it exactly offsets the quark’s gain of $+1e$.
$$ d^{-\frac13} \;\longrightarrow\; d^{-\frac13+1} + W^{-1} \;\longrightarrow\; u^{+\frac23} + W^{-1} $$
in other words,
$$ d \;\longrightarrow\; u + W^- $$
The unstable $W^-$ boson then decays almost instantly into an electron and an electron antineutrino:
$$ W^- \;\longrightarrow\; e^- + \bar{\nu}_e $$
The net result is the conversion of a neutron into a proton:
$$ n \;\longrightarrow\; p + e^- + \bar{\nu}_e $$
The process can be illustrated with a Feynman diagram:
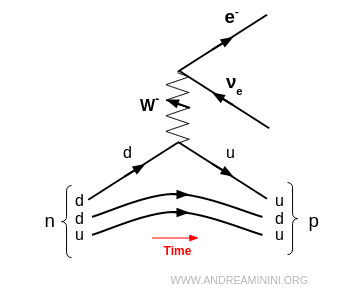
Here, an electron and an electron antineutrino are emitted. Together they constitute negative beta radiation (β⁻).
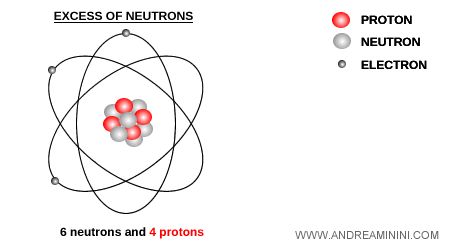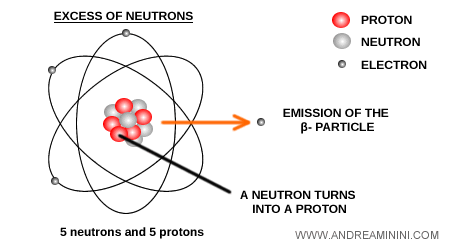
After decay, the atomic number $Z$ of the atom increases by one, since an extra proton is created, while the mass number $A$ remains unchanged because the total number of nucleons (protons + neutrons) stays the same.
β+ decay
When a nucleus has an excess of protons compared with neutrons, it can stabilize by converting a proton into a neutron.
How does a proton become a neutron?
This occurs when an up quark inside the proton transforms into a down quark.

When an up quark $(+\tfrac23 e)$ interacts with the weak field, it can transform into a down quark $(-\tfrac13 e)$, reducing its charge by $1e$.
To conserve the total charge of the system, a $W^+$ boson is emitted at the same time. Carrying a charge of $+1e$, it balances the $-1e$ loss of the quark.
$$ u^{\frac23} \;\longrightarrow\; u^{\frac23-1} + W^{+1} \;\longrightarrow\; d^{-\frac13} + W^{+1} $$
or simply,
$$ u \;\longrightarrow\; d + W^+ $$
The unstable $W^+$ boson decays almost instantly into a positron and an electron neutrino:
$$ W^+ \;\longrightarrow\; e^+ + \nu_e $$
What is a positron? A positron is the electron’s antimatter counterpart: it has the same mass as an electron but carries a positive charge. It is a particle of antimatter.
The overall outcome is the transformation of a proton into a neutron:
$$ p \;\longrightarrow\; n + e^+ + \nu_e $$
The process can also be shown with a Feynman diagram:
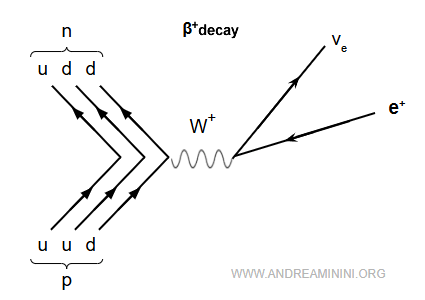
In this case, a positron and an electron neutrino are emitted. Together they form positive beta radiation (β⁺).

After the decay, the atom’s atomic number $Z$ decreases by one because a proton has been lost, while the mass number $A$ remains unchanged, since the total number of nucleons is the same.
Gamma radiation
In some beta decays, gamma radiation (γ) is also released.
This happens when, after the decay, the nucleons (protons and neutrons) in the nucleus are left in an excited state at a higher energy level.
When the nucleons drop back to their ground state, the excess energy is emitted as γ photons, high-energy particles of light.
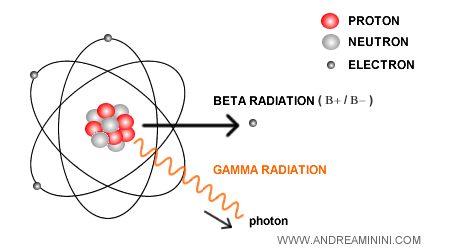
Note. This process is similar to the energy-level transitions of electrons in atomic orbitals, but in the case of nucleons the energy involved is much greater, since it is nuclear rather than electronic.
γ radiation is extremely energetic and far more penetrating than either alpha (α) or beta (β) radiation.
The role of neutrinos in beta decay
The energy carried away by the beta particle and gamma photons does not account for the full energy released by the decay.
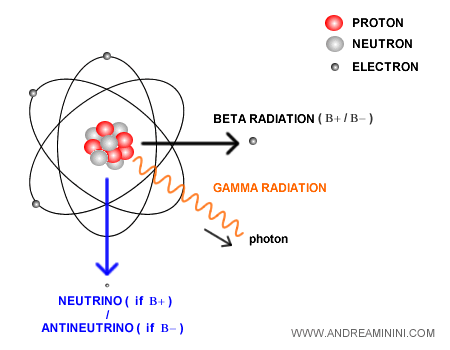
The “missing” energy is carried off by another fundamental particle: the neutrino.
The neutrino is a subatomic particle with almost zero mass and no electric charge. It is always produced in beta decay to conserve the total energy and momentum of the system.
There are two main types:
- Electron neutrino ($\nu_e$), emitted in β⁺ decay.
- Electron antineutrino ($\bar{\nu}_e$), emitted in β⁻ decay, with properties opposite to those of the neutrino.
An example of a nuclear reaction
The following nuclear reaction illustrates the beta minus decay of Thorium-234. The nucleus emits an electron ( e- ), while a neutron converts into a proton. An antineutrino ( v ) is also released.
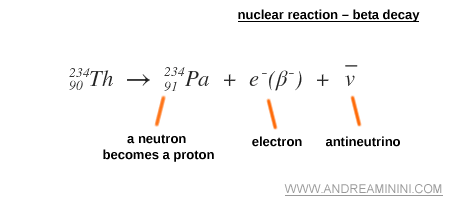
The resulting nucleus is no longer an isotope of thorium ( Th ) but of protactinium ( Pa ), because the nucleon composition has changed and the atomic number increases from 90 to 91, while the atomic mass remains at 234. The end product is therefore a different chemical element.
